Do you ever wonder what makes up the world around you?
Everything you can think of is made up of one or more elements — the chemical building blocks of the universe.

The periodic table helps you understand how these elements behave in both experiments and the natural world.
If you know how to read the periodic table, you'll be able to quickly figure out an element's characteristics...and have a much better chance at passing your science classes!
What's The Periodic Table?
The periodic table is a chart that:
Shows natural and artificial elements in the environment.
Arranges elements in groups and periods.
Helps you predict the properties and behaviors of different elements.
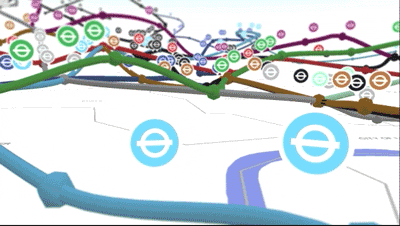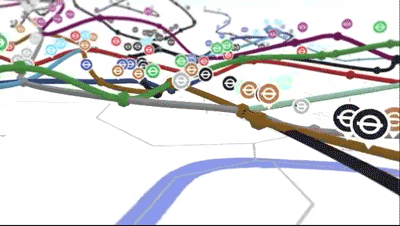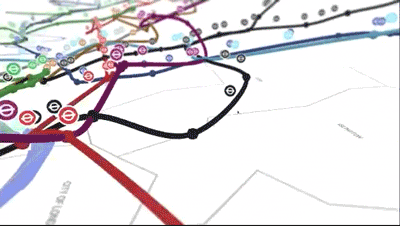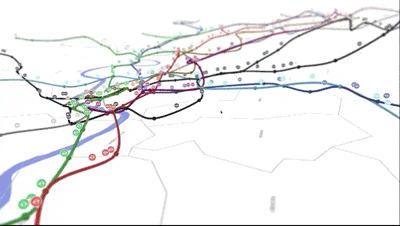
💡 Think of the periodic table as amapof the elements.
If you know how to read a subway map, you can get around a city.
It's the same with the periodic table: you can better navigate chemistry, biology, and physics class if you know how to read it!
Elements
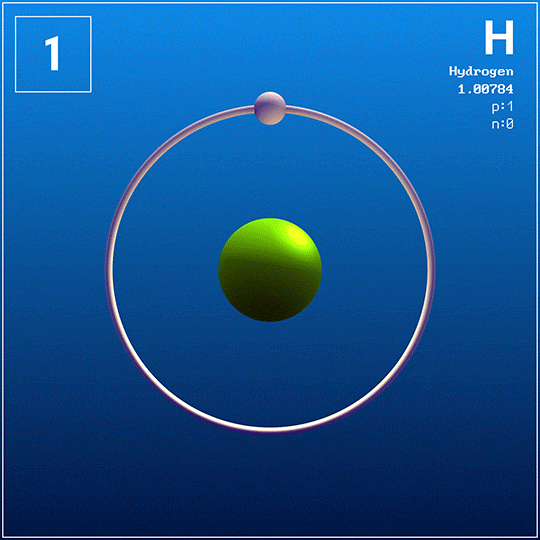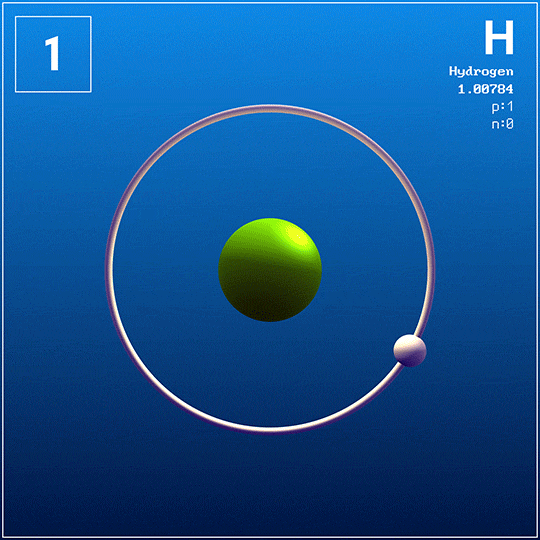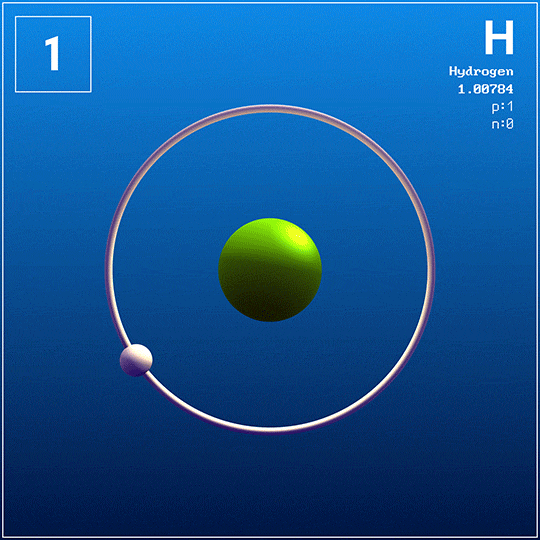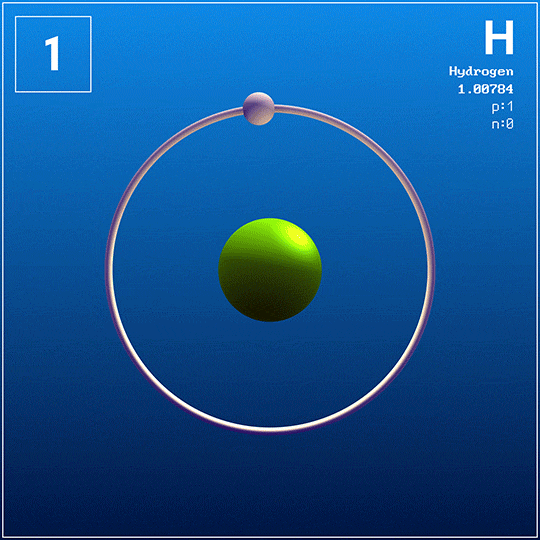
Elements appear as squares in the periodic table.
Each square contains information about the element:
Atomic number — the number of protons in the element's atoms.
Atomic symbol — a shortened name of the element.
Atomic mass — the average weight of the element's atoms.
Reading an element square is like looking up information about a place you want to visit on a city map.
Just like when you use Google Maps to find the address of a site you want to visit, you can use a square on the periodic table to learn basic information about an element.
Element Square Example
Let's look at lithium (Li), a metal used in phone and car batteries.

Atomic Symbol = a shortened version of the name of an element
It's represented as one or two letters in the middle of the square.
Lithium's atomic symbol is Li.
Atomic Number = the number of protons in the element's atoms
It's located at the top left of the square.
Lithium's atomic number is 3, so it has 3 protons.
Atomic Mass = theaverage weight of an atom in an element
It's represented as a decimal at the bottom of the square.
Lithium's atomic mass is 6.941.
Knowledge Check 1
Compare these two elements:
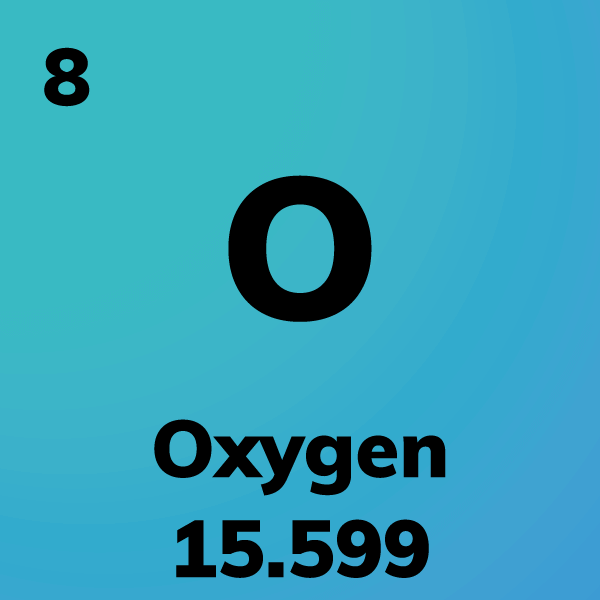
Oxygen makes up most of the air we breathe.
Carbon is an element that exists in all living things.

Quiz
Select all of the true statements below:
Groups
Groups appear as columns numbered from 1-18, from left to right at the top of the table.
All the elements in each group have the same number of outer electrons, which means they have similar atomic structures and similar behaviors.
Think of groups as specific regions you want to visit on a trip.
If two cities are in the same country or area, the people there will probably speak the same language and have similar customs.
It's the same idea with two elements in a group: if they have the same number of outer electrons, they'll react to other elements in a similar way.
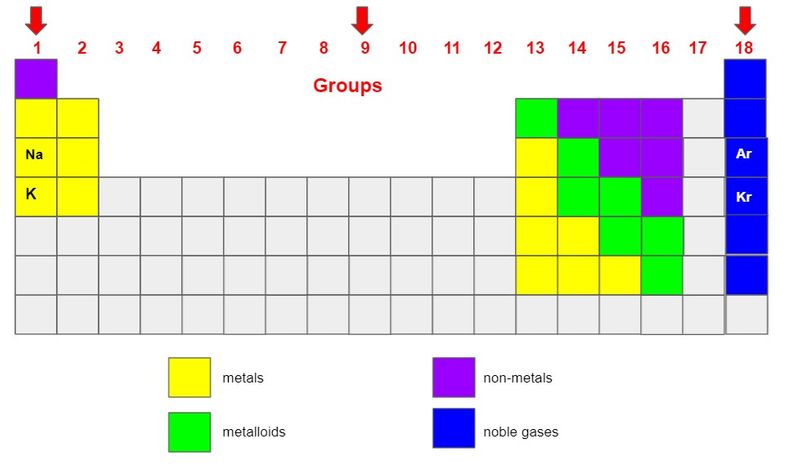
Sodium (Na) and potassium (K) are both in Group 1 so they have a few things in common:
They're minerals found all over the Earth.
They boil and melt at lower temperatures than other elements.
They float in water but quickly dissolve.
They're silvery-white in color.
They're essential for keeping humans alive.
Sodium helps balance fluids in your body.
Potassium helps your muscles and nerves function.
Periods
Periods appear as rows numbered from 1-7, from top to bottom on the left side of the table.
All elements in each period have the same physical properties .
Think of periods as places with similar climates on a map.
If you visit two tropical places, they'll both be hot year-round.
Similarly, two elements in the same period have similar physical characteristics.
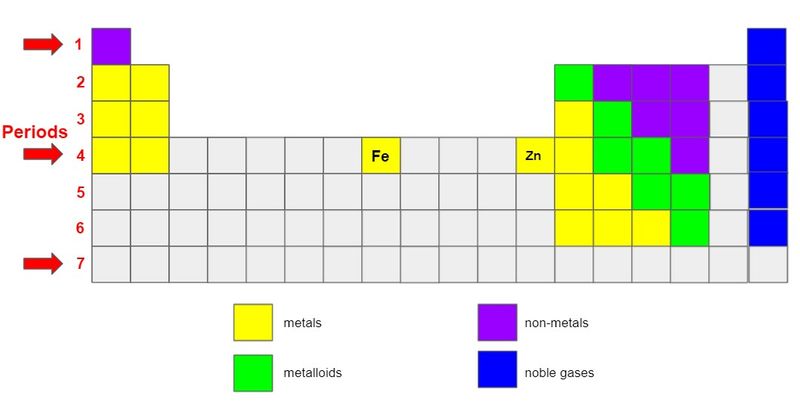
Iron (Fe) and zinc (Zn)are in Period 4. What do they have in common?
They're good conductors of heat.
They can be shaped or bent.
They're important to manufacturing because they make steel stronger and more durable.

Knowledge Check 2
Take a look at the periodic table below:
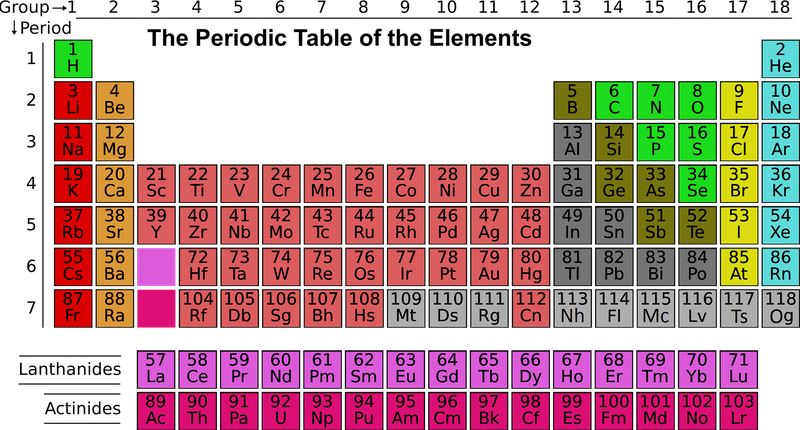
Quiz
Which elements have the same physical properties as potassium (K)?
Take Action
Now that you know the basics of the periodic table, kick your studies up a notch!
You don't need to know every element, but getting some practice with the periodic table will help you become more familiar with the elements you need to know in class.
Your feedback matters to us.
This Byte helped me better understand the topic.


