
This logo isn't an ad or affiliate link. It's an organization that shares in our mission, and empowered the authors to share their insights in Byte form.
Rumie vets Bytes for compliance with our
Standards.
The organization is responsible for the completeness and reliability of the content.
Learn more
about how Rumie works with partners.
If you’ve been inside a science classroom, chances are you’ve seen the periodic table of elements.
It’s also a popular nerdy symbol — you might find it on a mug or a t-shirt, too!
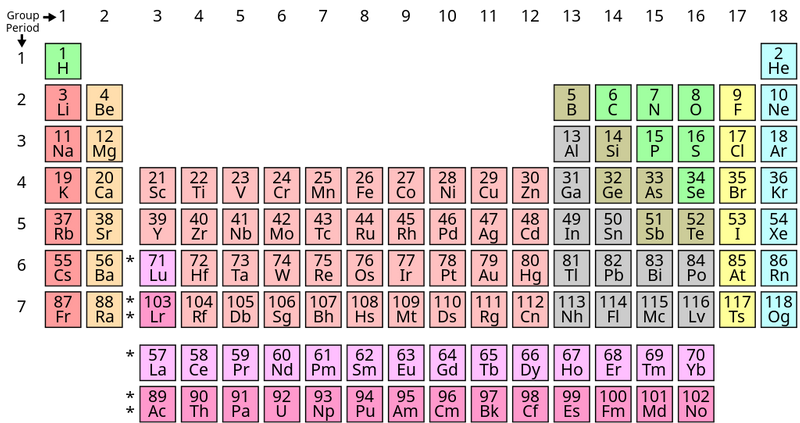 Image by Offnfopt via Wikimedia Commons.
Image by Offnfopt via Wikimedia Commons.
Click here for an accessible version of the period table. 👈
But have you ever wondered why the periodic table looks the way it does?
It’s not a typical table with even rows and columns — it has breaks and gaps, and looks like a castle.
 Photo by Nabih El Boustani on Unsplash
Photo by Nabih El Boustani on UnsplashWhat if I told you that weird shape is intentional, and even helpful when you are studying chemistry?
Organizing the Elements
A lot of the periodic table's shape comes from its construction.
Dmitri Mendeleev was a Russian scientist who created the first periodic table of elements.
 Image from Brady Haran, CC0, via Wikimedia Commons
Image from Brady Haran, CC0, via Wikimedia Commons
What's special about his work is how he organized the elements into a meaningful pattern:
Elements are ordered by number of protons and increasing mass.
 Once you know how to read the periodic table's layout, you, too, can understand the huge amount of information it contains!
Once you know how to read the periodic table's layout, you, too, can understand the huge amount of information it contains!
Reading the Periodic Table
The periodic table is read left to right, top to bottom. Some rows have gaps (like row 1) to line up elements into columns and rows based on their properties.
Though it's not a perfect grid, the periodic table can be read like a map. That means we can use periods and rows like coordinates to locate elements.
Locating an Element
If you know what period and group an element is in, you can locate it exactly on the periodic table.
For example, lithium is the only element in both period 2 and group 1!

The simplest definitions of periods and groups: they're rows and columns on the periodic table.
But there's so much information you can tell about an element based on which period and group it's a part of. Let's take a closer look!
Quiz
Review the periodic table above. What element is located in period 4, group 6?
Remember that the period is the row and the group is the column. Chromium is correct because it's in the fourth row (period 4) and the 6th column (group 6). Hafnium is the reverse, in period 6 and group 4. Potassium is in period 4 but group 1. Carbon is atomic number 6 and is located in period 2, group 14.
Periods = Rows
Rows on the table are called periods — that's where the name "periodic table of elements" comes from. Periodic here means "occurring at regular intervals."
Many elements in the same period have similar physical properties, and patterns of those properties occur at regular intervals — they're periodic!
 Examples of Properties:
Examples of Properties:
From left to right, across a period:
Metallic character (being shiny and formable) decreases
Number of protons in the nucleus increases
Atomic radius (how big the atom is) decreases

Example: Sashary wants to know more about the properties of nickel (Ni). She looks at the periodic table and sees that iron (Fe) is in the same period as nickel (Ni). She knows that iron is shiny, silvery metal and (correctly) concludes that nickel is similar because they are both in period 4.

Groups = Columns
A group of elements are in the same column, reading from top to bottom, and are labeled 1-18.
Elements in the same group have similar reactivity — they react with other elements in similar ways.
 Example: Rafael wants to know more about the element bromine (Br). He looks at the periodic table and sees that chlorine (Cl) is in the same group as bromine (Br). He knows chlorine is very reactive because he's a lifeguard and adds chlorine to the pool to kill germs. He correctly assumes that bromine is also very reactive since it's in group 17 with chlorine.
Example: Rafael wants to know more about the element bromine (Br). He looks at the periodic table and sees that chlorine (Cl) is in the same group as bromine (Br). He knows chlorine is very reactive because he's a lifeguard and adds chlorine to the pool to kill germs. He correctly assumes that bromine is also very reactive since it's in group 17 with chlorine.

Periods vs. Groups
Now that you have a deeper understanding of periods and groups on the periodic table, let's summarize:
Periods
Horizontal rows
7 total
Elements have similar physical properties like: metal/nonmetal, size, number of protons
Groups
Vertical columns
18 total
Elements have similar reactivity —chance they'll have a chemical reaction
Quiz
Shiloh wants to know more about element 27, cobalt (Co). Which of these elements will cobalt be similar to, and why?
Elements have the most in common with others in their same period and group. Shiloh should consider what they know about iron and iridium since those are in the same period or group. The other elements aren't in the same period or group as cobalt, so they likely have less in common.
Take Action
Nice work! Now you know that periods are rows, and groups are columns that organize elements into the periodic table based on their properties.
 That means you're ready to learn even more about chemistry:
That means you're ready to learn even more about chemistry:
This Byte has been authored by
Andrea Stewart
Learning Designer
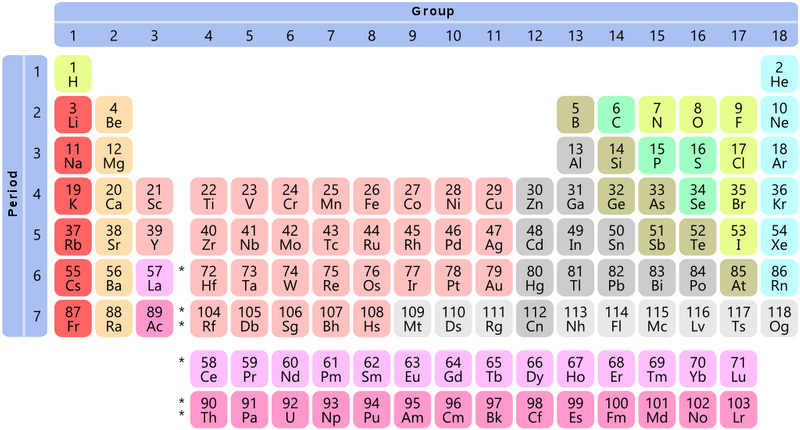 Image by
Image by