This logo isn't an ad or affiliate link. It's an organization that shares in our mission, and empowered the authors to share their insights in Byte form.
Rumie vets Bytes for compliance with our
Standards.
The organization is responsible for the completeness and reliability of the content.
Learn more
about how Rumie works with partners.
Why is balancing chemical equations important in chemistry?

Balancing chemical equations helps you understand the relationship between reactants and products in a chemical reaction.
It also helps you satisfy the law of conservation of mass. This law states that mass is neither created nor destroyed — atoms on the reactant and product sides must have the same mass and number.

Learn simple strategies that will have you balancing chemical equations, like putting the sameamount of substances on each side of the balance scale.
What you need to know
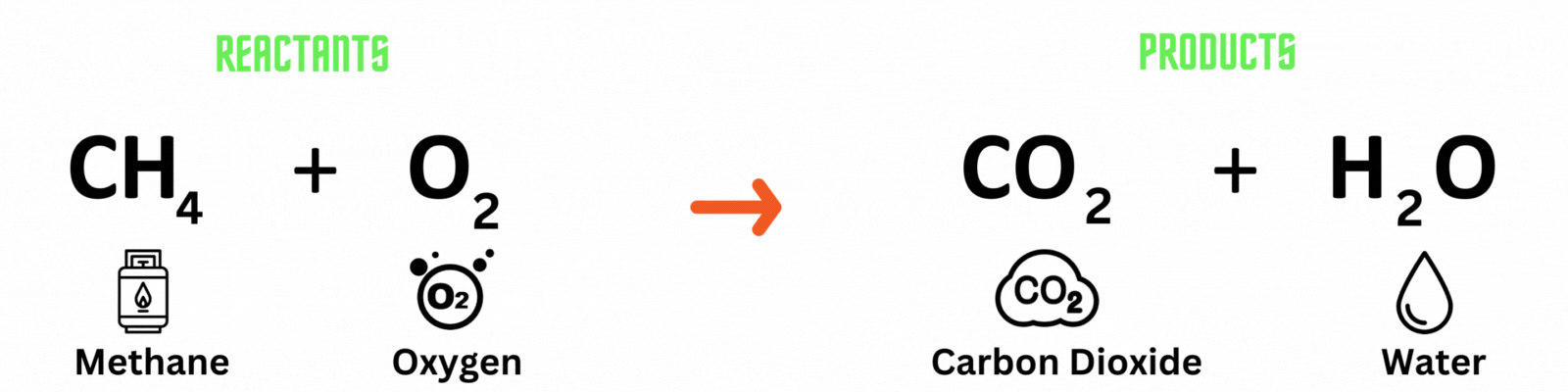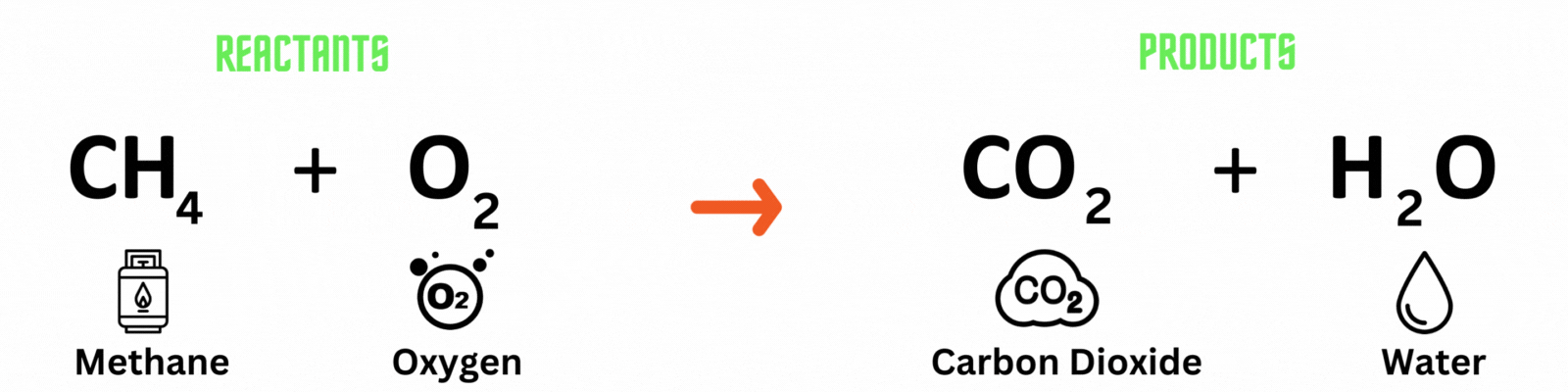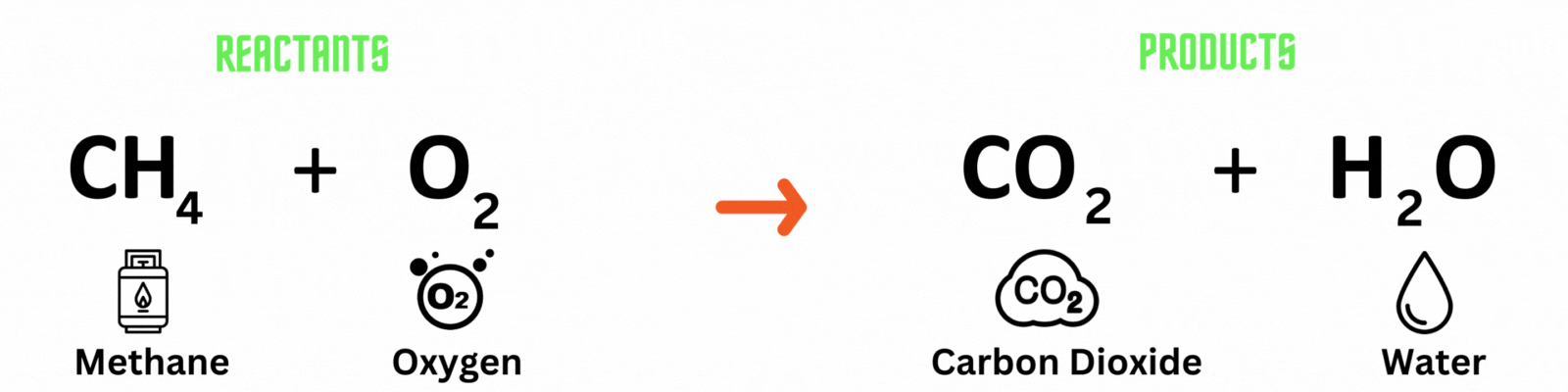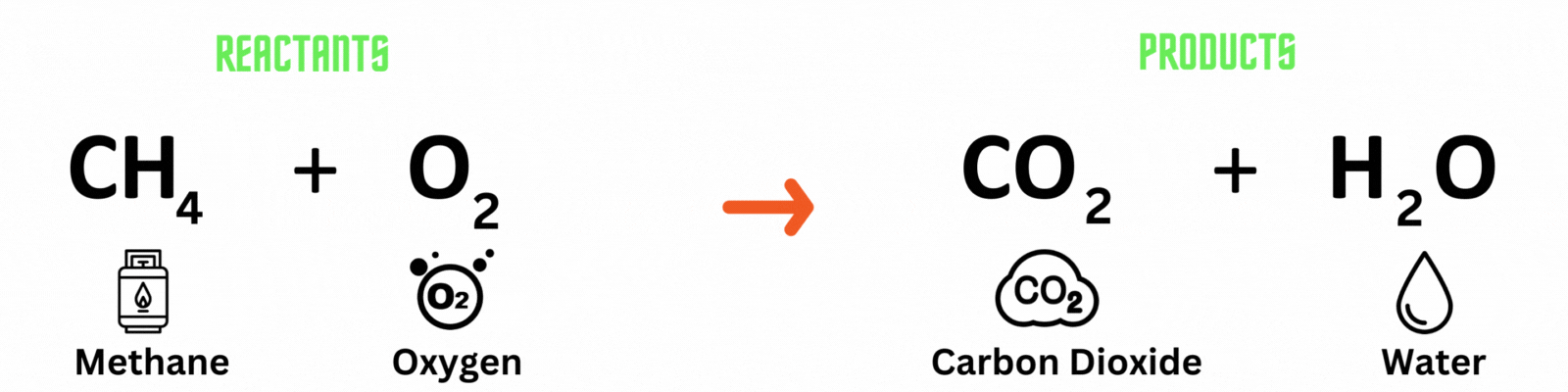
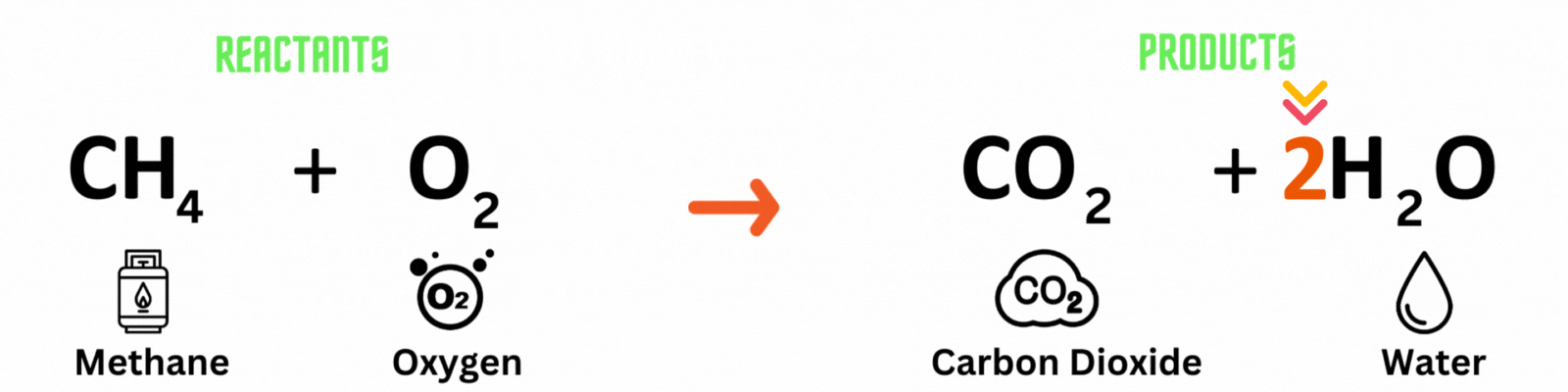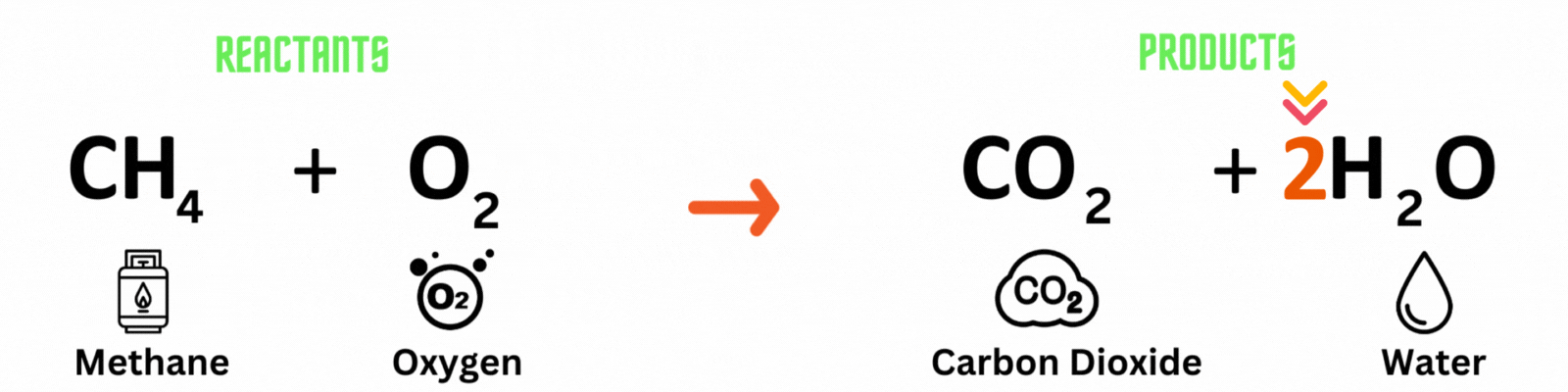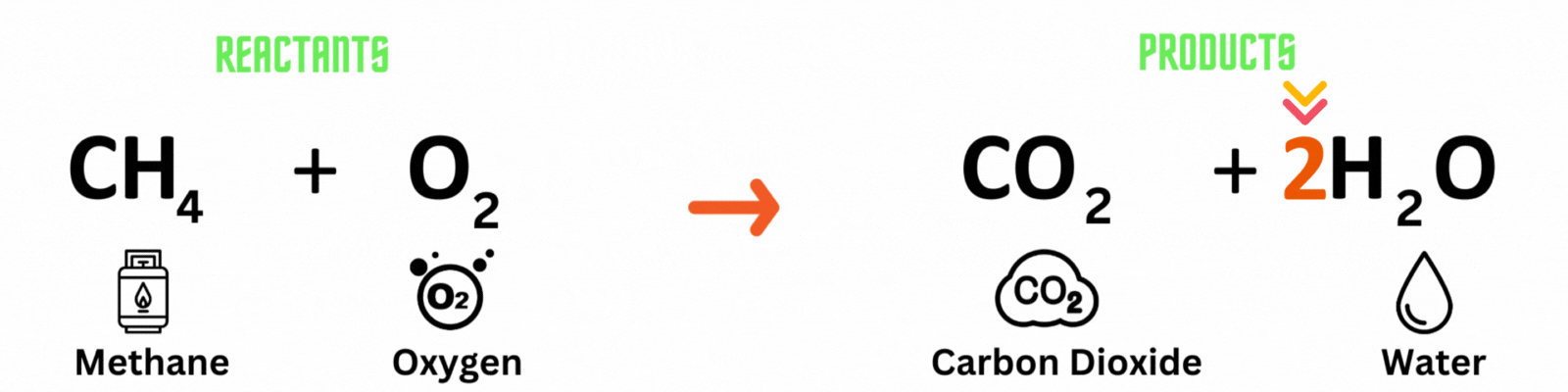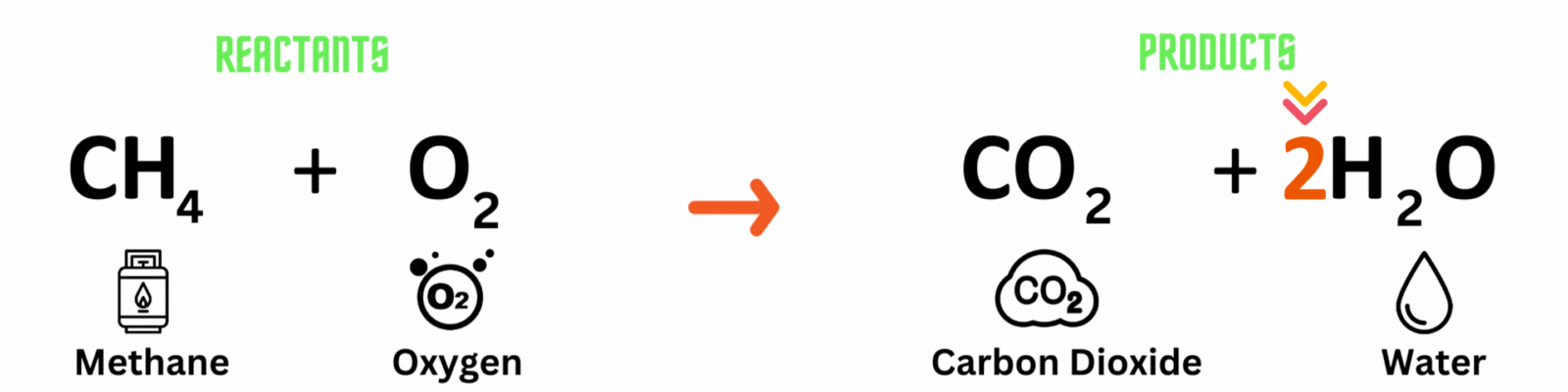
Chemical equation: a visual representation of a chemical reaction. It has 3 parts.
Reactants: substances present before the chemical reaction.
Products: substances present after the chemical reaction.
Step 1: Count the number of atoms
Reactants
Carbon atom (C) = 1
Hydrogen atom (H) = 4
Oxygen atom (O) = 2

Products
Carbon atom (C) = 1
Hydrogen atom (H) = 2
Oxygen atom (O) = 2 + 1 = 3

This is NOT a balanced chemical equation! There are unequal numbers of atoms on both sides of the equation.
Quiz
By looking at the chemical equation above, which element(s) have unequal numbers of atoms on reactant and product sides of the equation? Select all that apply:
There are 4 hydrogen atoms on the reactant side and 2 on the product side. Also, there are 2 oxygen atoms on the reactant side and 3 on the product side.
Step 2: Change the coefficient of the substance(s)
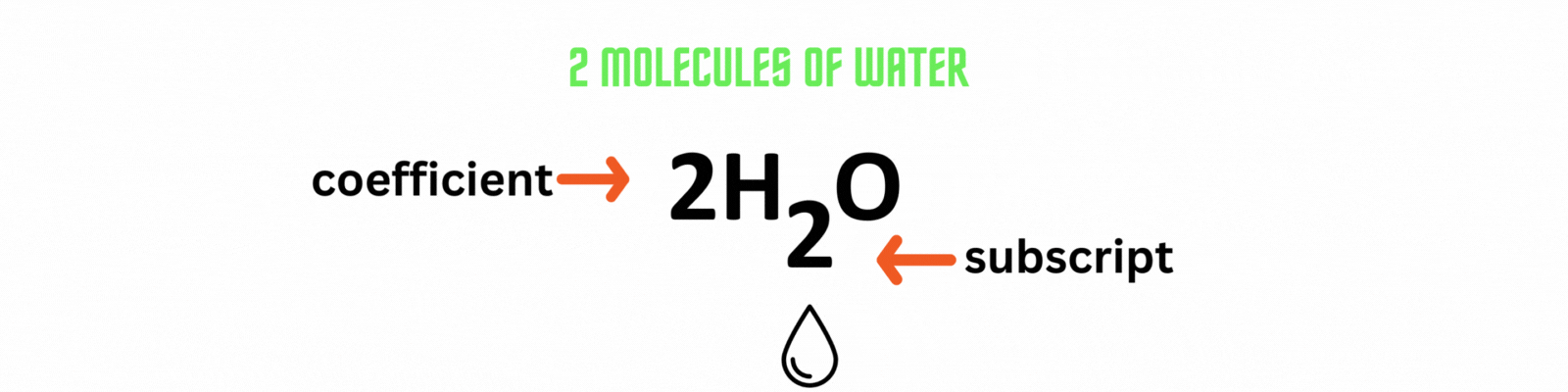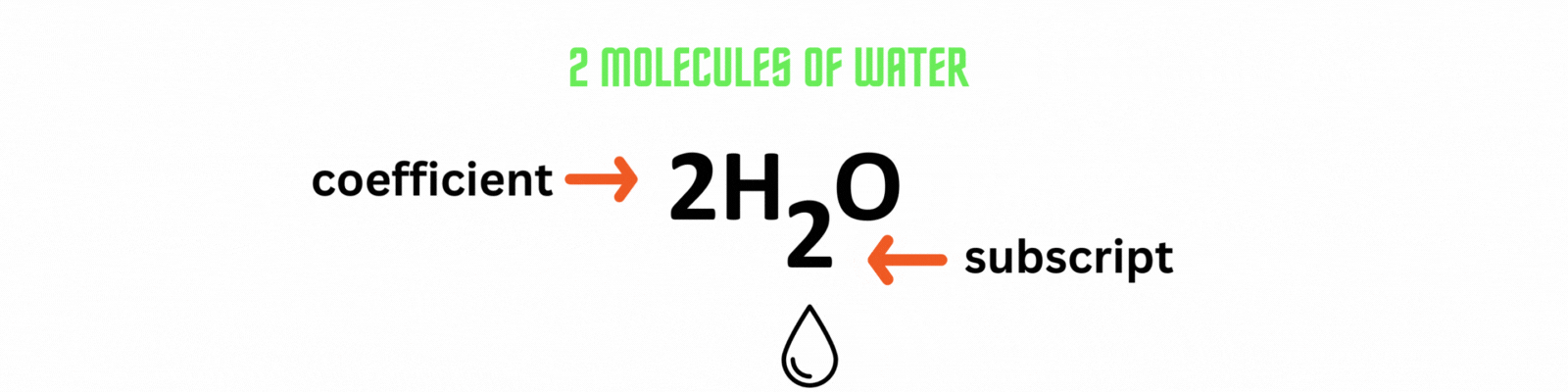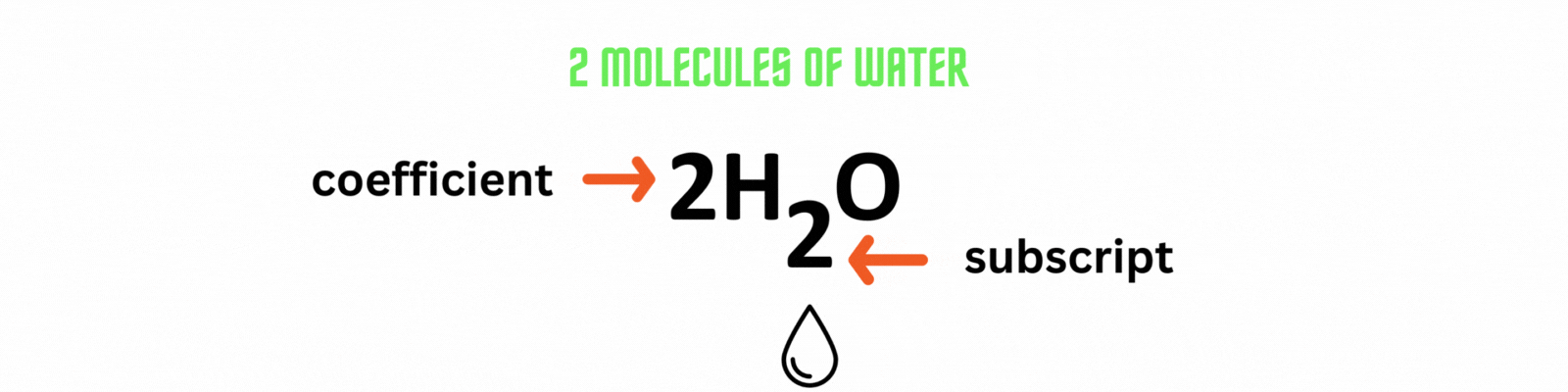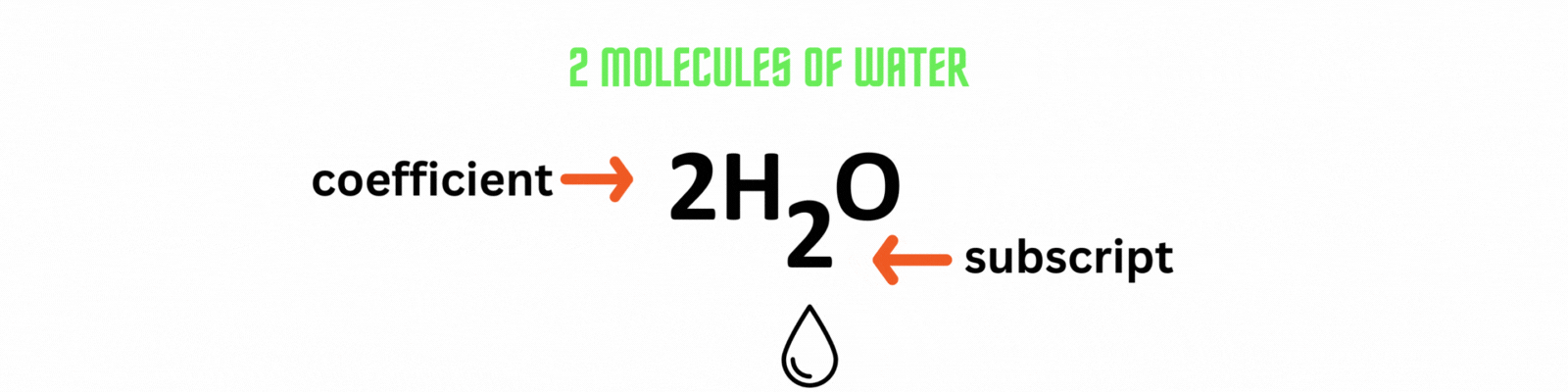
Place the coefficient 2before the water molecule on the product side then count the number of atoms on each side. ✍🏼
Do's:
Add the coefficient before an element or a compound to balance out a chemical equation. Use the least common multiple between numbers as the coefficient.
Reactants
Carbon atom (C) = 1
Hydrogen atom (H) = 4
Oxygen atom (O) = 2

Products
Carbon atom (C) = 1
Hydrogen atom (H) = 2 x 2 = 4
Oxygen atom (O) = 2 + (2 x 1) = 4

This is NOT a balanced chemical equation yet! There are unequal numbers of oxygen atoms on each side of the chemical equation.
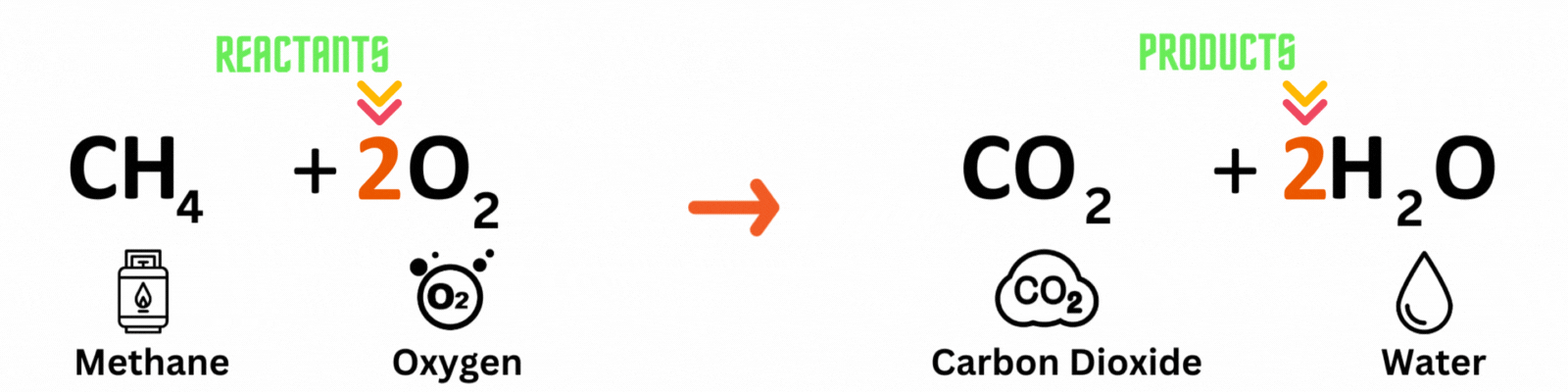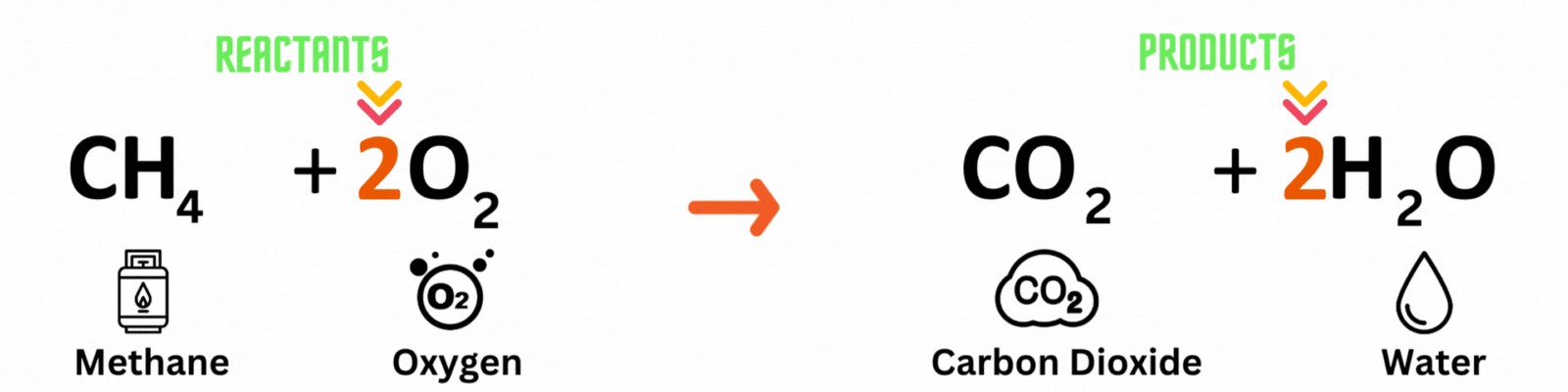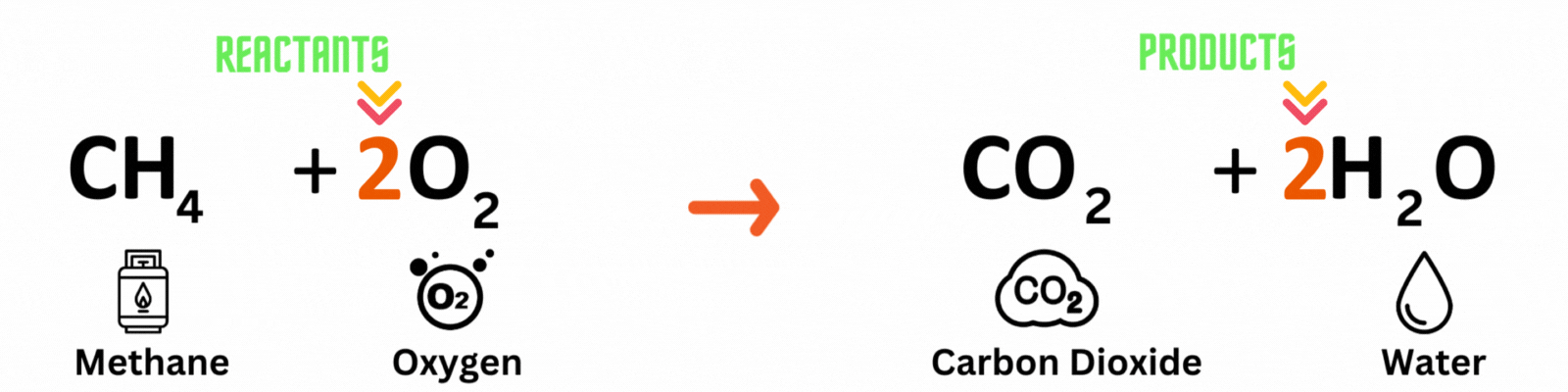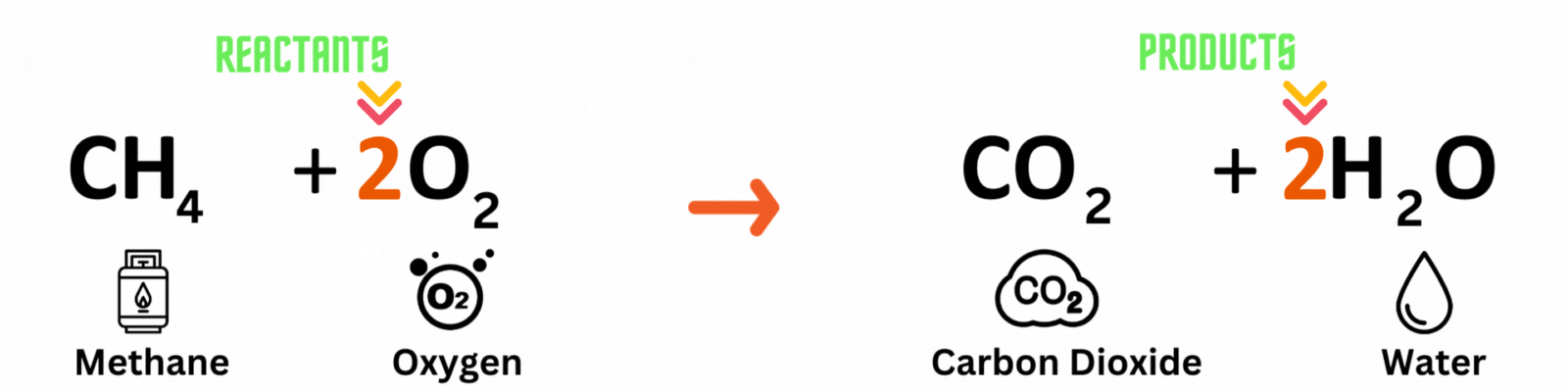
Step 3: Repeat until you reach a balanced equation
Place another coefficient 2 before oxygen on the reactant side, then count the number of atoms on each side again. Make sure both the reactant and product sides have the same number of atoms. ✍🏼
Do's:
Use whole numbers when balancing a chemical equation.
Keep changing the coefficient until all elements have the same numbers on both sides. A good rule of thumb is to balance out elements that appear multiple times last. In this equation, you'll balance out oxygenlast because it pops up in two molecules on the product side.
Reactants
Carbon atom (C) = 1
Hydrogen atom (H) = 4
Oxygen atom (O) = 2 x 2 = 4

Products
Carbon atom (C) = 1
Hydrogen atom (H) = 2 x 2 = 4
Oxygen atom (O) = 2 + (2 x 1) = 4

This is a BALANCED chemical equation! All number of atoms for carbon (C), hydrogen (H), and oxygen are equal.
Practice your new skill ✍🏼
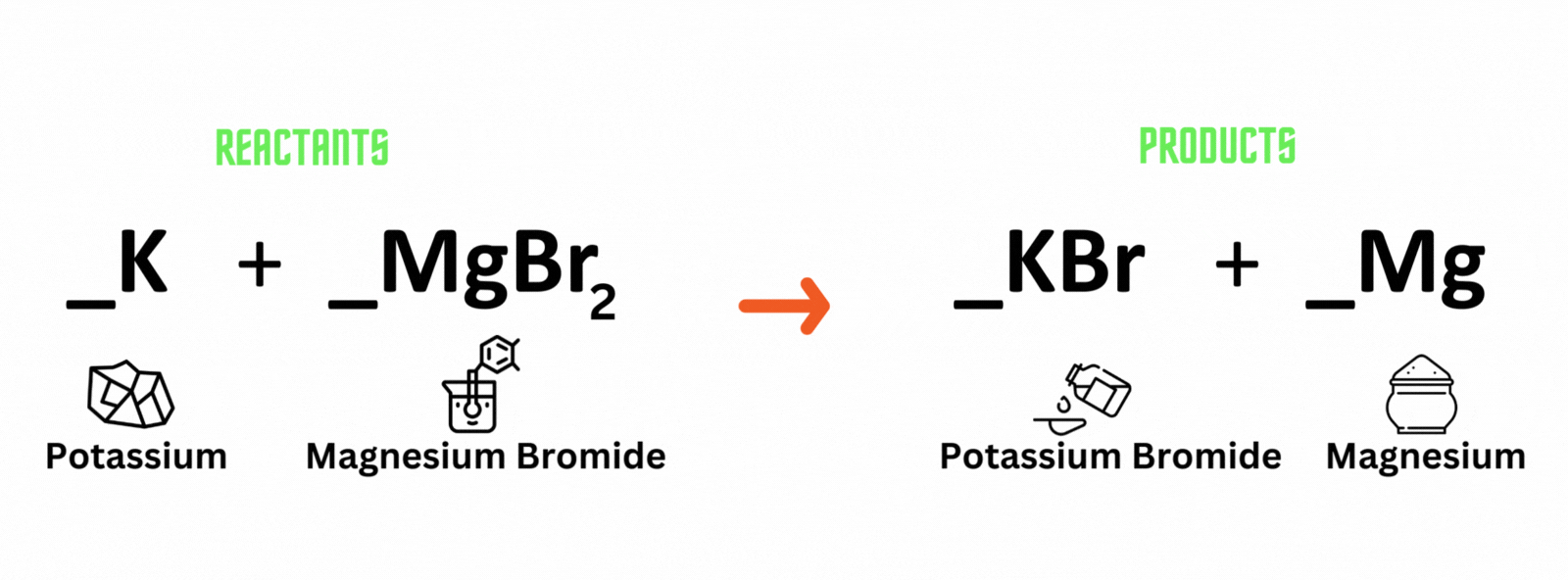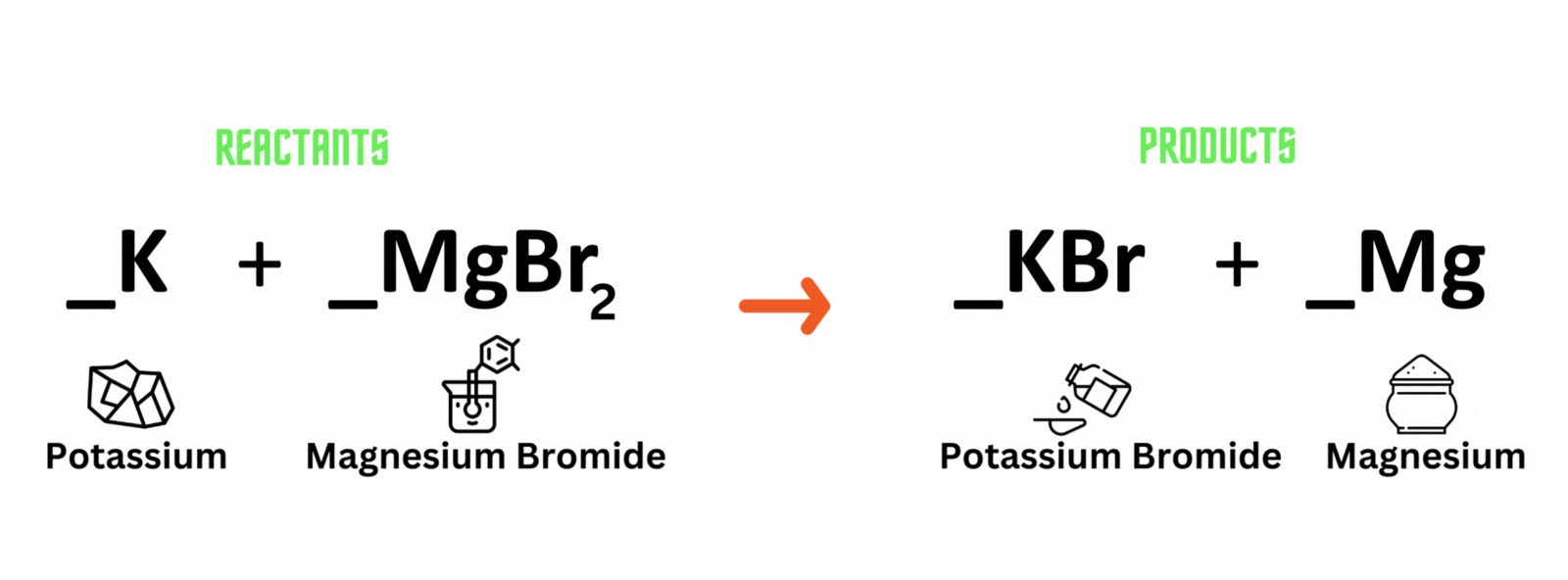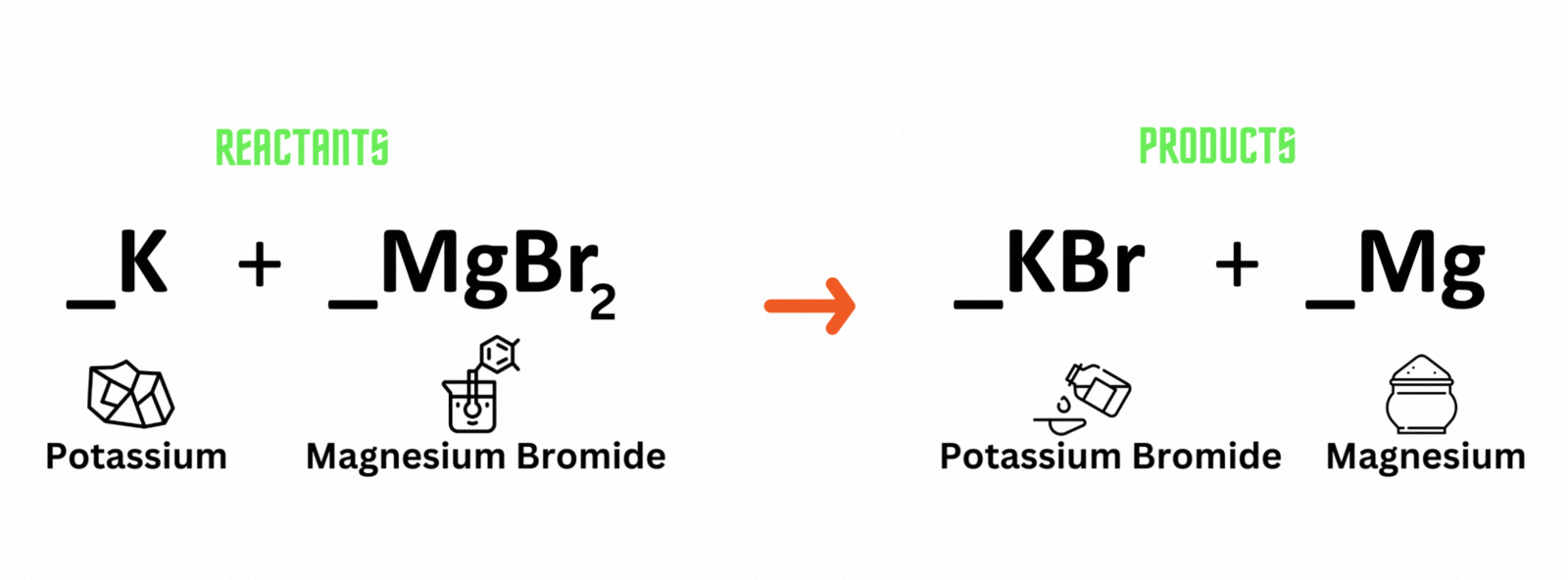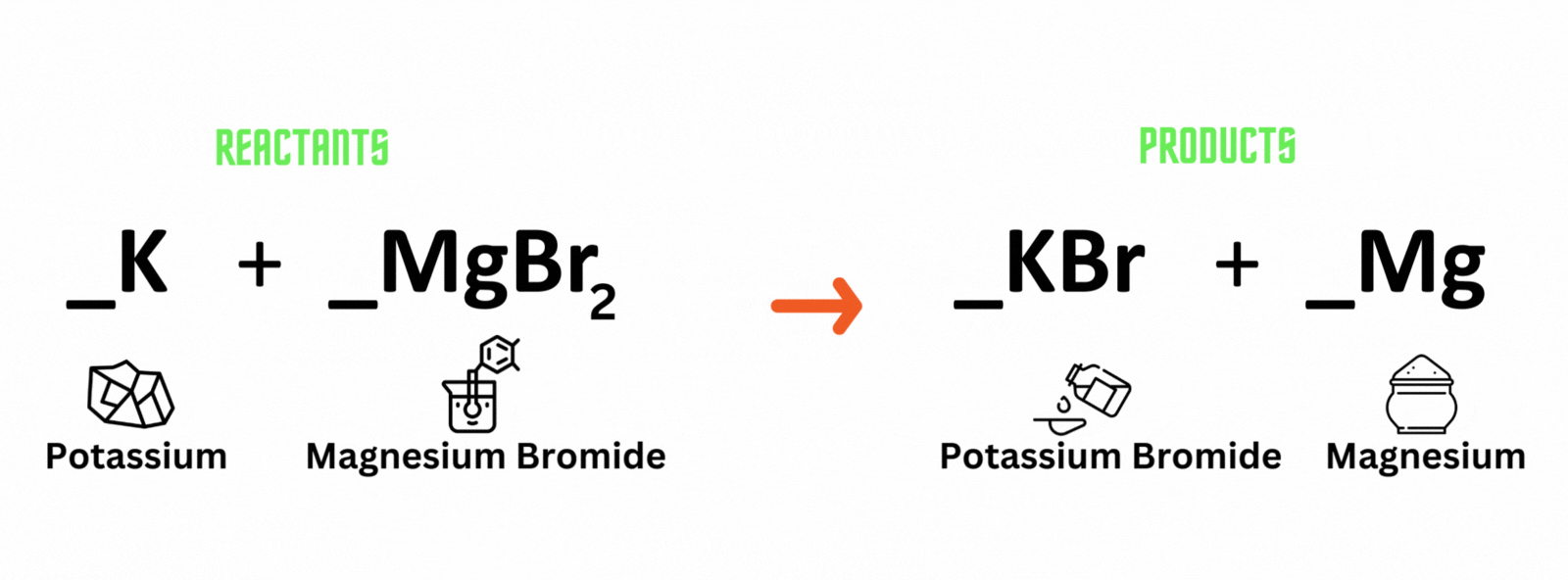
Potassium reacts with magnesium bromide, which produces potassium bromide and magnesium. Where should you place coefficient 2on the reactant and product sides to balance the equation?
A. Potassium (K) and potassium bromide (KBr)
B. Magnesium bromide (MgBr) and magnesium (Mg)
C. Magnesium bromide (MgBr) and potassium bromide (KBr)
D. Potassium (K) and magnesium (Mg)
Quiz
Which letter above is the best answer?
Place coefficient 2 before potassium (K) on the reactant side, and another 2 before potassium bromide (KBr) on the product side to balance the equation.
Take Action

Balancing a chemical equation is a trial-and-error method. Counting the atoms and changing the coefficient until you reach a balanced equation is necessary. Try these activities and polish your new skills:
This Byte has been authored by
Rowena Turcios
Educator | Learning & Curriculum Designer
BSE, MA