
This logo isn't an ad or affiliate link. It's an organization that shares in our mission, and empowered the authors to share their insights in Byte form.
Rumie vets Bytes for compliance with our
Standards.
The organization is responsible for the completeness and reliability of the content.
Learn more
about how Rumie works with partners.
You're about to teach your students the foundation of science: atomic basics.
While this topic is the basis of chemistry, it can be difficult for students to grasp because atoms can't be seen with the naked eye. How can you help your students understand atomic basics so they can expand and apply them throughout the year?
Dissect atomic basics by helping students understand what an atom is, its basic structure, and what it does.
What students should know #1: What is an atom?
An atomis the smallest unit of matter. They make up everything in the universe, from things you can see (like tables) to things you can't (air). Atoms are made up of mostly empty space and have a nucleus with electrons surrounding it.
All atoms are made of the same subatomic particles (protons, neutrons, electrons), but they are unique in the number of protons they have. The number of protons determines what element it is.
A particular element is made up of one type of atom. For example, if you have hydrogen (an element), it will be made of the same kind of atom.
Teacher's guide #1: Help students understand what an atom is

What resources and activities can help students understand atomic basics?

Teaching resources
Videos: Introduce your students to the world of atoms with visuals, such as "The 2,400-year search for the atom — Theresa Doud" and "Just How Small is an Atom?", both by TED-Ed.
Lesson: Help your students understand how atoms make up an element! "Exploring Elements" is a lesson developed by the American Association of Chemistry Teachers and provides activities and handouts that will help students explore elements using the periodic table. Be sure to adjust the lesson to your students' current understanding.
Creative activities
Paper-cutting: This teaches students how small an atom can be. Have students fold and cut a strip of paper as many times as possible. If you use a 11"x1" strip, to get to the size of an atom, you need to divide it about 31 times. Use this as an example of how small atoms are! Here is a sample handout by Quarked! you can use.
Match atoms to elements: This activity can help students see the relationship between atoms and elements. Instruct students to draw an atom for an element. For example, ask students to draw an oxygen atom. Their model should have eight protons, eight neutrons, and eight electrons. Do the reverse and ask students to label an atom based on the number of protons, neutrons, and electrons to review the elements!
What students should know #2: What is the structure of an atom?
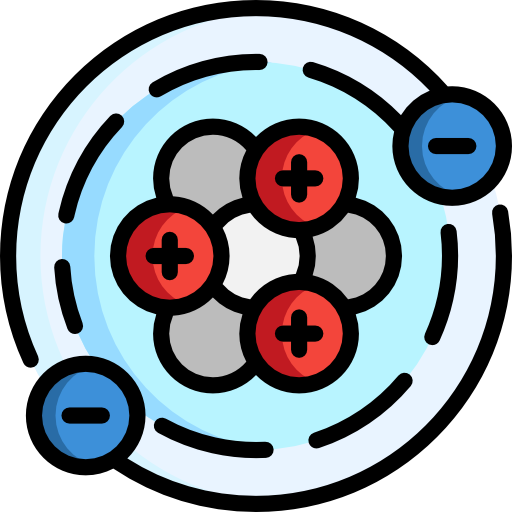
Atoms are made of three subatomic particles: protons, neutrons, and electrons. A proton is a subatomic particle with a positive charge and determines what element it is. For example, if an atom has three protons, the element is lithium (the third element of the periodic table).
A neutron has no charge (neutral). An electron has a negative charge and bonds with other atoms. In a neutral element (the most "natural" state), the number of protons and electrons is equal.

Protons and neutrons are bound in the nucleus, found in the center of the atom. They are sometimes called nucleons because they make up the nucleus. Electrons are much smaller than protons and neutrons, and they're found in shells surrounding the nucleus.
Teacher's guide #2: Help students understand an atom's structure
 What resources and activities can help students understand an atom's structure?
What resources and activities can help students understand an atom's structure?
 Teaching resources
Teaching resources
Use an image/GIF: Use a model that helps students understand where subatomic particles are located. For example, use a GIF like this one:
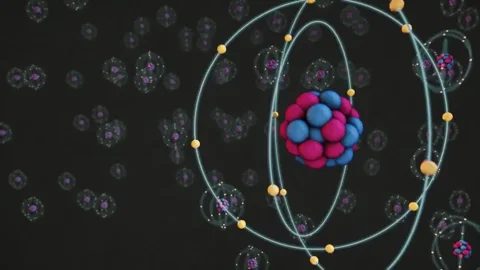
In the GIF, the protons are pink, the neutrons are blue, and the electrons are yellow. The nucleus has the protons and neutrons, and the electrons are orbiting around the nucleus.
Use a simulation: Simulations are an interactive way for students to explore a topic! PBS' simulation "Atomic Structure" simplifies what an atomic structure is. This is a great resource to learn about subatomic structures starting from a pencil and going all the way down to the atom!
 Creative activity
Creative activity
Create a model of the atom: This is a fun way for students to create an atomic model! You can use a paper plate with colored beads, pom poms, pieces of construction paper, or even small, colorful candies to represent the subatomic particles. You can also create a model using a pipe cleaner with beads.
Create a simulation: Simulations are not just for teachers! Let your students build their own atoms using PhET's "Build an Atom" simulation. What makes this simulation great is that students can see how the net charge and mass number change when they add or take away specific subatomic particles.
Below is an idea for a quiz question that you could ask your students to check their understanding of an atom's structure. Try it yourself!
Quiz
Jenny is working on a mystery element that has 2 protons. What can she conclude? Select all answers that apply:
The element has two protons, so it matches with the second element of the periodic table: helium. This is not beryllium because beryllium (the 4th element) has 4 protons. The number of protons match the number of electrons, so there are two electrons. The term "nucleons" refer to protons and neutrons collectively, so identifying the element as having two nucleons would be incorrect.
Did you know?
What students should know #3: What does an atom do?
Atoms bond with each other to create everything in the universe. They form bonds to create new elements by changing the chemical composition of the atom.
Changing the chemical composition, such as the number of protons or electrons an atom has, affects how it reacts. For example, two hydrogen atoms and an oxygen atom form water as H2O.

Atoms can break bonds and cause reactions that help drive processes in our world, such as when plants use sunlight to perform photosynthesis. Atoms also react to each other. Depending on its composition and bonds, it can give a substance specific characteristics, such as being flammable!
Teacher's guide #3: Help students understand what an atom does
 What resources and activities can help students understand what an atom does and how it works?
What resources and activities can help students understand what an atom does and how it works?
Teaching resources
Use real-world examples: Increase learner interest by making it relatable. For example: "Wood is flammable. Flammability is a change of an atom's bonds. When something like wood burns, bonds that hold the wood together are broken and rearranged to create carbon dioxide and water, which release energy in the process."

Creative activity
Atomic bonding challenge: This is a fun, interactive way to teach your students about covalent and ionic bonding! Developed by the Teaching Channel, the Atomic Bonding Challenge, students will have paper plates with valence electrons punched out with a hole punch. The goal is for students to find a peer whose plate will help them create a full valence shell.

Take Action
Atoms are the building blocks of chemistry, but they're really the heart of science. Without atoms, nothing would exist or occur, which is why helping students understand what they are is so important. These ideas and resources can expand their knowledge of the atom to understand how the universe works and what makes them, them!

Blow your students' minds by helping them understand atomic basics:
This Byte has been authored by
Emily Nguy
Science Teacher